CareStart™
COVID-19
Antigen
Rapid Diagnostic Test for the Detection of SARS-CoV-2 Antigen

Access Bio’s CareStart™ COVID-19 Antigen
RAPID POC TEST
The CareStart™ COVID-19 Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens directly collected from individuals who are either suspected of COVID-19 by their healthcare provider within the first five days of symptom onset or from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., inpatient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
NOTE: For Serial Screening of asymptomatic individuals. The serial screening indication is only applied to products manufactured by Access Bio Inc. after April 12, 2021. The product batches listed in the Official Notification Letter (click to see) should not be marketed or used for POC serial screening purposes.
- Lateral flow assay
- Detect SARS-CoV-2 nucleocapsid protein antigen
- Rapid results within 10-15 minutes
- Intended at POC setting (i.e., in patient care settings) by medical professionals operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
- For use under the Emergency Use Authorization (EUA) only
- For in vitro diagnostic use only
- For prescription use only
- For use with direct anterior nasal or nasopharyngeal swab specimens
Clinical Performance
- 93.75% PPAa and 99.32% NPAb when used with nasopharyngeal swab
- 87.18% PPAa and 100% NPAb when used with anterior nasal swab
TEST PRINCIPLES
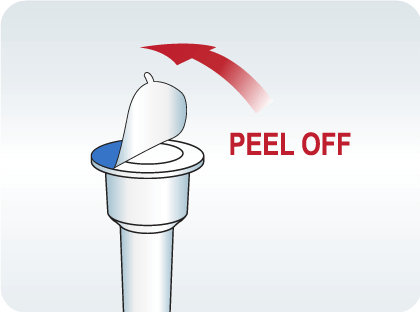
Peel off aluminum foil seal from the top of the extraction vial containing the extraction buffer.
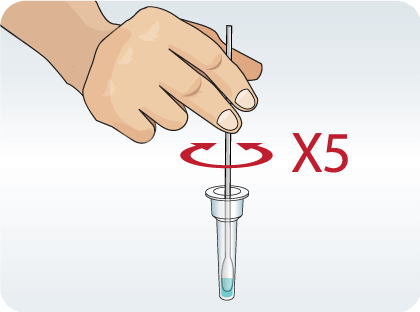
Place the swab into the extraction vial. Rotate the swab vigorously at least 5 times.
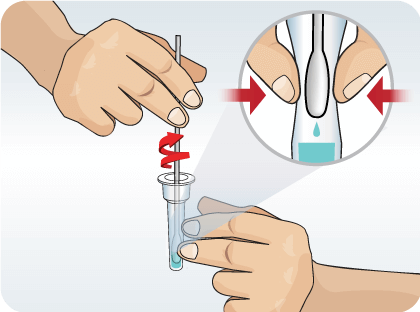
Remove the swab by rotating against the extraction vial while squeezing the sides of the vial to release the liquid from the swab. Properly discard the swab.
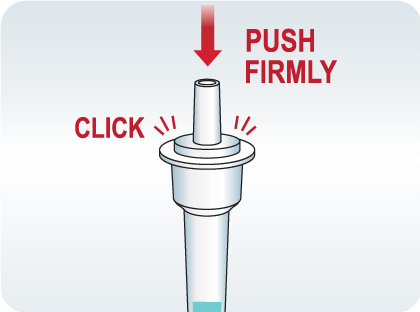
Close the vial by pushing the cap firmly onto the vial.
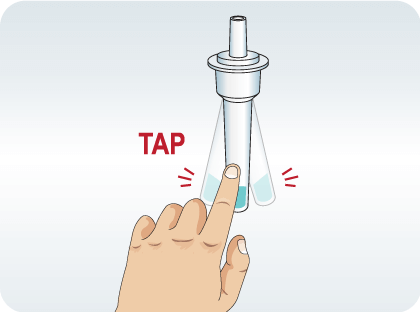
Mix thoroughly by flicking the bottom of the tube.
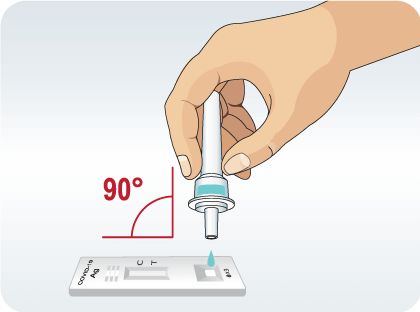
Invert the extraction vial and hold the sample vertically above the sample well. Squeeze the vial gently. Allow three (3) drops of sample to fall into the sample well.
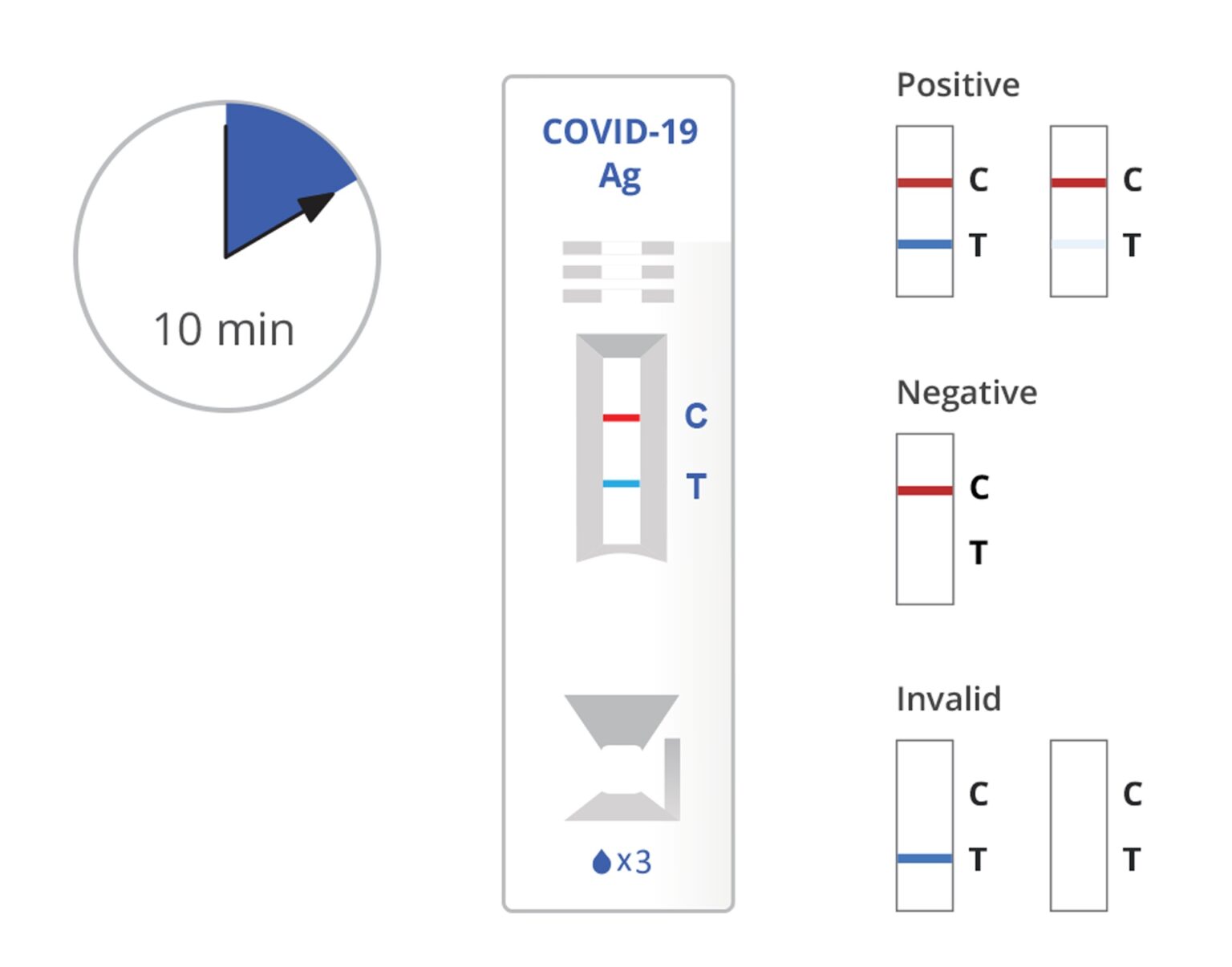
Read the result at 10 minutes. The test result should not be read after 15 minutes.
Warning: The false positive, false negative, or invalid results may occur if the test is interpreted outside of the interpretation window.
Positive: SARS-CoV-2 antigen present; does not rule out co-infection with other pathogens. The color intensity in the test region will vary depending on the amount of SARS-CoV-2 antigen present in the sample. Any faint colored line(s) in the test region(s) should be considered positive.
Negative: Negative results are presumptive. Negative test results do not preclude infection and should not be used as the sole basis for treatment or other patient management decisions, including infection control decisions, particularly in the presence of clinical signs and symptoms consistent with COVID-19, or in those who have been in contact with the virus. It is recommended that these results be confirmed by a molecular testing method, if necessary for patient management.
Invalid: If the red-colored line in the control region “C” is not visible, the result is invalid. Re-run the test one time using the remaining specimen in the extraction vial if an invalid result is obtained during initial testing.
- 20 TEST DEVICES
- 20 ASSAY BUFFER
- 20 EXTRACTION VIALS AND CAPS
- 20 SPECIMEN COLLECTION SWABS
- 1 POSITIVE AND 1 NEGATIVE CONTROL SWAB
- 1 INSTRUCTIONS FOR USE

