CareStart™
COVID-19
lgM/lgG
Rapid Diagnostic Test for the Detection of SARS-CoV-2 IgM/IgG Ab

Access Bio’s CareStart™ COVID-19 lgM/lgG
RAPID COVID-19 TESTING TOOL
NOW FDA EMERGENCY USE AUTHORIZED
The CareStart™ COVID-19 IgM/IgG is an immunochromatographic lateral flow assay intended for the qualitative detection and differentiation of Immunoglobulin M (IgM) and Immunoglobulin G (IgG) antibodies to SARS-CoV-2 in human serum, plasma (sodium citrate, sodium heparin, or dipotassium EDTA), and venous whole blood (sodium citrate, sodium heparin, or dipotassium EDTA).
The CareStart™ COVID-19 IgM/IgG is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The CareStart™ COVID-19 IgM/IgG should not be used to diagnose or exclude acute SARS-CoV-2 infection. This testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform moderate or high complexity tests.
- For use under Emergency Use Authorization only
- For in vitro diagnostic use only
- For prescription use only
- Detect and differentiate IgM/IgG antibodies specific to SARS-CoV-2
- Requires small sample volume (10 uL of venous whole blood, serum, or plasma)
- Results available within 10-15 minutes
- No equipment required
- Identify and monitor an individual’s previous infection history and immune response to COVID-19
Clinical Performance (NIH/NCI)
- IgM Sensitivity and Specificity: 90% and 98.8%
- IgG Sensitivity and Specificity: 100% and 98.8%
Clinical Performance against comparable method
- IgM PPAa and NPAb: 89.06% and 99.45%
- IgG PPAa and NPAb: 96.88% and 99.45%
- aPPA: Positive Percent Agreement, bNPA: Negative Percent Agreement
TEST PRINCIPLES
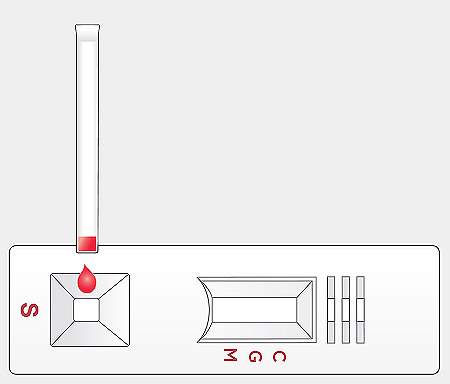 Transfer venous whole blood, serum, or plasma sample:
Transfer venous whole blood, serum, or plasma sample:
- Using a provided blood transfer pipette: Press the top part of the provided blood transfer pipette and touch the sample by the pipette tip while pressing the pipette. Releasing the press slowly to fill the pipette with sample up to the blue marked line (approximately 10 µl). Add the blood sample to the sample well “S” of the test device by pressing the top part of the blood transfer pipette.
- Using a micropipette: Transfer 10 µl of the venous whole blood, serum, or plasma sample to the sample well “S” of the test device using a micropipette.
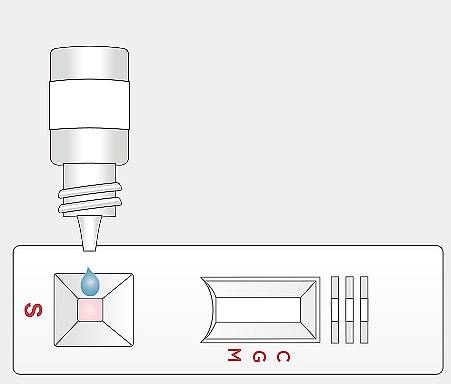 Open the cap and invert the assay buffer bottle and hold vertically above the sample well. Squeeze the bottle gently to add one (1) drop of the assay buffer solution to the sample well “S” immediately after sample loading.
Open the cap and invert the assay buffer bottle and hold vertically above the sample well. Squeeze the bottle gently to add one (1) drop of the assay buffer solution to the sample well “S” immediately after sample loading.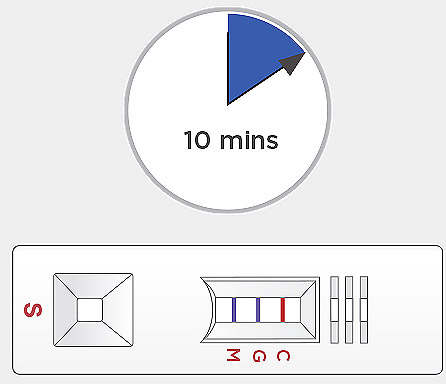 Start a timer. Read the result at 10 minutes. The test results should not be read earlier than 10 minutes. Test results should not be read after 15 minutes.
Start a timer. Read the result at 10 minutes. The test results should not be read earlier than 10 minutes. Test results should not be read after 15 minutes.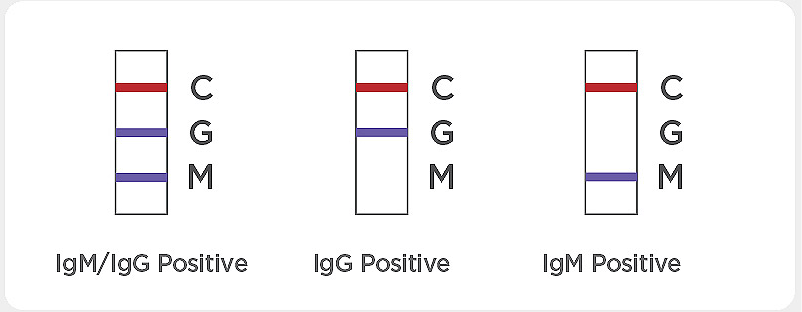
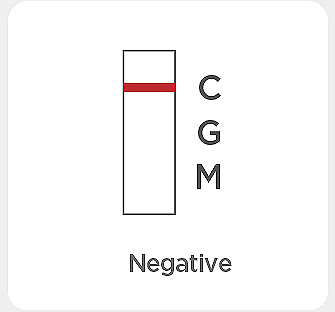
Result with faint colored line(s): The color intensity in the test region will vary. Any faint colored line(s) in the test region(s) should be considered positive.
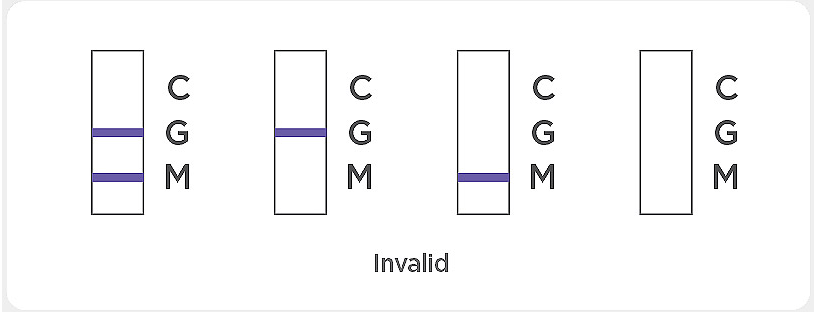
If the control line “C” is not visible, the result is invalid. Re-run the test using a new test device. If the same invalid result persists, contact the manufacturer or distributor before continuing to test samples.
- 25 TEST DEVICES
- 1 ASSAY BUFFER
- 25 BLOOD TRANSFER PIPETTES
- 25 ALCOHOL SWABS
- 25 STERILE SAFETY LANCET
- 1 INSTRUCTION FOR USE
- 1 QUICK REFERENCE INSTRUCTIONS (QRI)

