CareStart™
COVID-19
MDx RT-PCR
COVID-19 DETECTION KITS

Access Bio’s CareStart™ COVID-19 MDx RT-PCR
AN AMERICAN SOLUTION FOR RELIABLE
COVID-19 TESTING
NOW FDA EMERGENCY USE AUTHORIZED
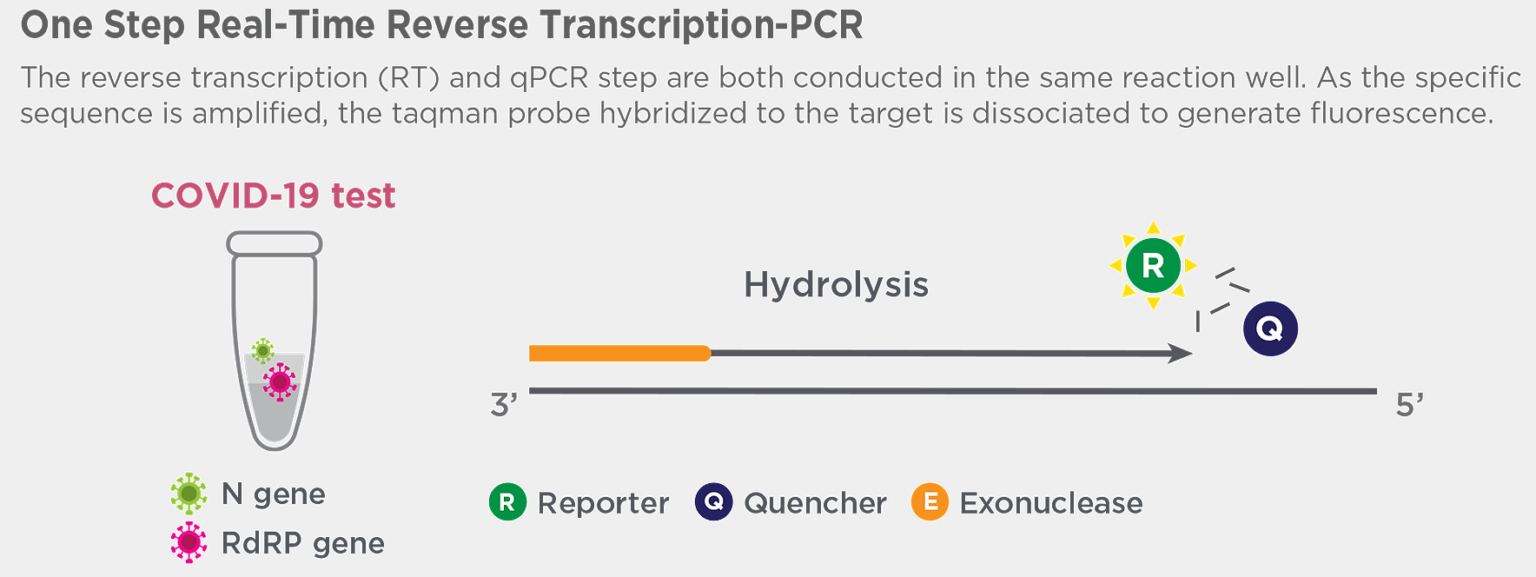
The CareStart™ COVID-19 MDx RT-PCR is a real-time reverse transcription polymerase chain reaction (RT-PCR) test intended for the qualitative detection of nucleic acid from SARS-CoV-2 in respiratory specimens (such as nasopharyngeal, oropharyngeal and nasal swabs, and nasopharyngeal wash/aspirate or nasal aspirate) and bronchoalveolar lavage from individuals suspected of COVID-19 by their healthcare provider.
Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet requirements to perform high complexity tests.
- For use under an Emergency Use Authorization (EUA) only
- For in vitro diagnostic use only
- Rx Use only
- For the qualitative detection of human coronavirus SARS-CoV-2 viral RNA extracted from respiratory tract specimens
- Administered via nasopharyngeal, oropharyngeal and nasal swabs and nasopharyngeal wash/aspirate or nasal aspirate, and bronchoalveolar lavage
- Processing time is less than 83 minutes
Available for use with the following lab systems:
- CFX96 Touch™ system (Bio-Rad Laboratories, Inc.)
- Applied Biosystems 7500/7500 Fast Real-Time PCR System(Thermofisher Scientific)
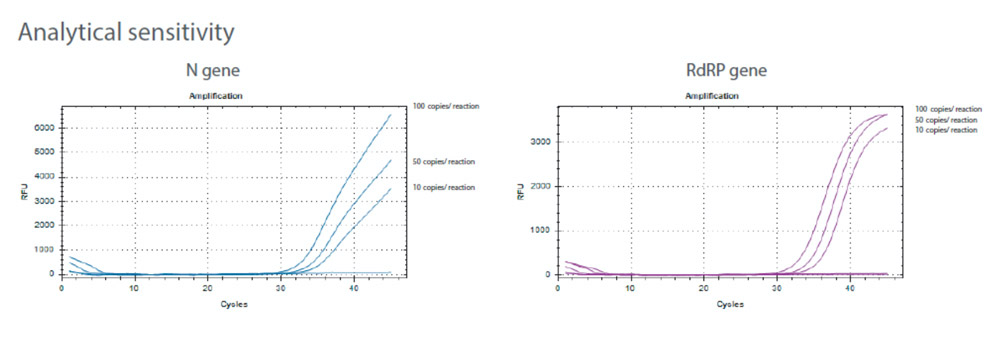
Clinical Performance
- 100% PPAa and 100% NPAb
ONE STEP REAL-TIME REVERSE TRANSCRIPTION PCR TEST METHOD
SPECIFICATIONS AND TECHNICAL INFORMATION