CareStart™
COVID-19
Antigen Home Test
Self Test for SARS-CoV-2 Antigen Detection
Intended Use
The CareStart™ COVID-19 Antigen Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigens from SARS-CoV-2.
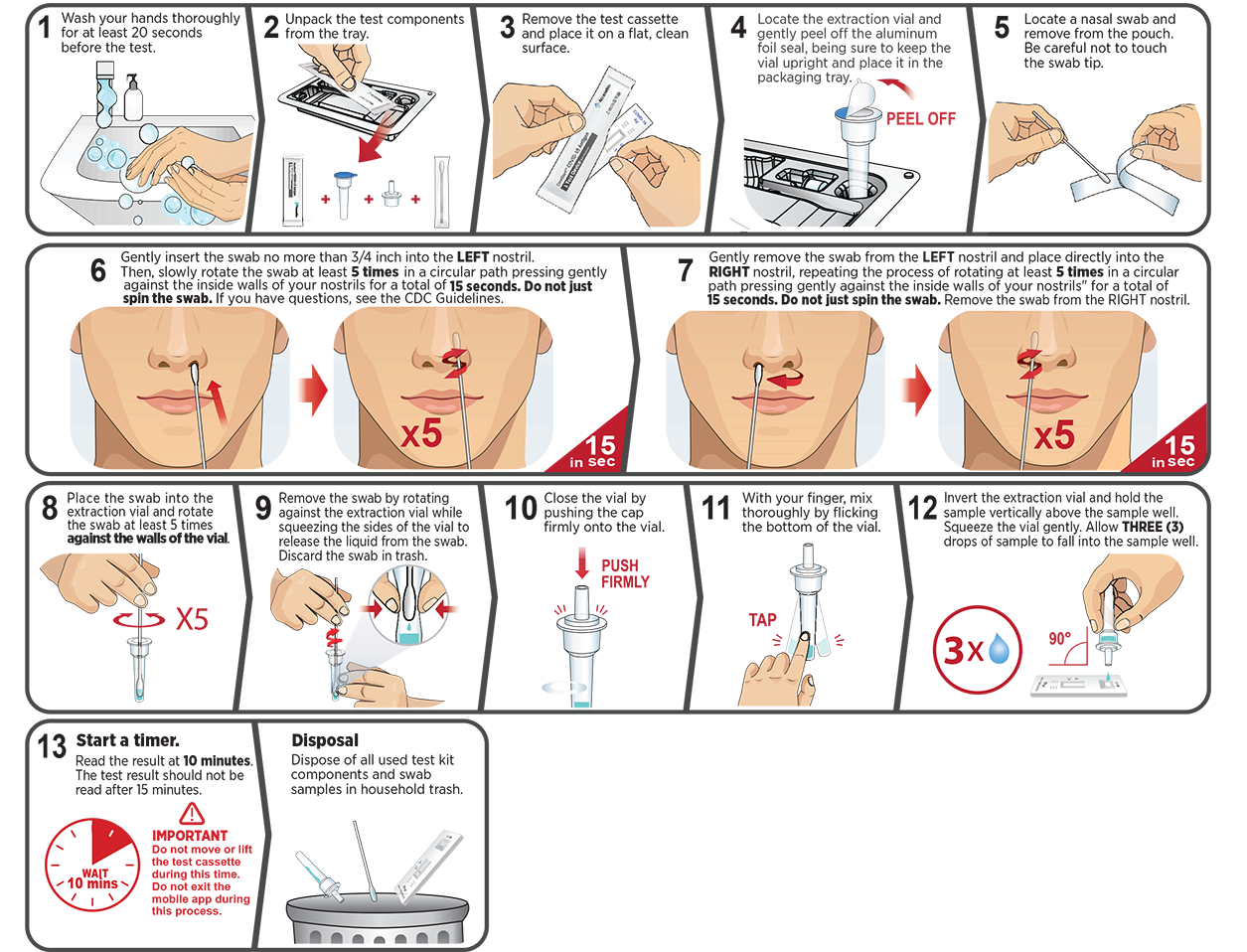
This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older with symptoms of COVID-19 within the first 7 days of symptom onset. This test is also authorized for non-prescription home use with adult-collected nasal (nares) swab samples from individuals aged 2 years or older with symptoms of COVID-19 within the first 7 days of symptom onset.
This test is also authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older, or adult-collected anterior nasal (nares) swab samples from individuals aged 2 years or older, with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
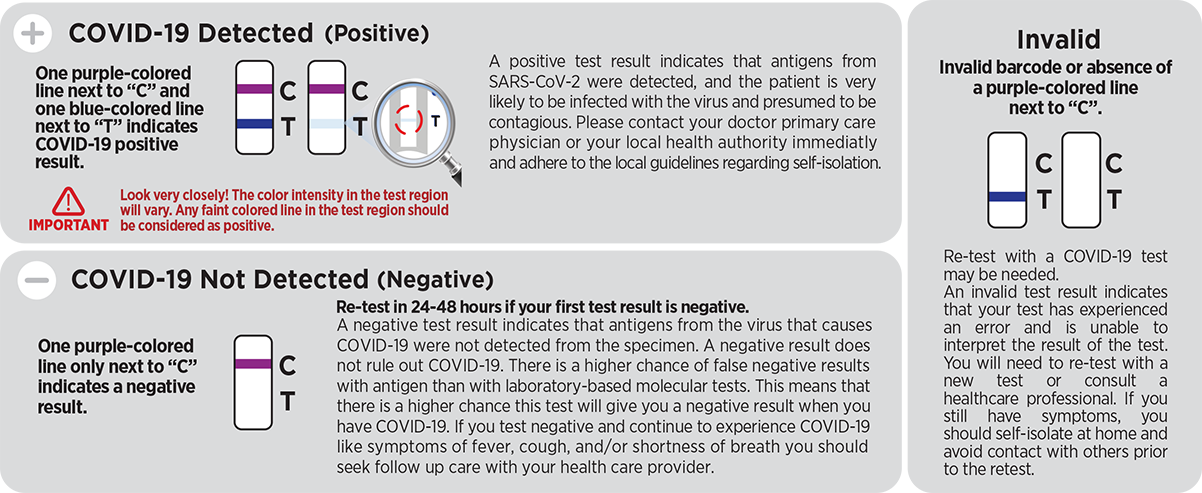
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. The antigen is generally detectable in anterior nasal (nares) swab specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses and the agent detected may not be the definite cause of disease. Individuals who test positive with the CareStart™ COVID-19 Antigen Home Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary.
Negative results should be treated as presumptive and confirmation with a molecular assay for patient management, may be performed if necessary. Negative results do not rule out SARS-CoV-2 infection, and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of an individual’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19.
For serial testing programs, additional confirmatory testing with a molecular test for negative results may be necessary, if there is a high likelihood of COVID-19, such as, an individual with a close contact with COVID-19 or with suspected exposure to COVID-19 or in communities with high prevalence of infection. Additional confirmatory testing with a molecular test for positive results may also be necessary, if there is a low likelihood of COVID-19, such as in individuals without known exposures to COVID-19 or residing in communities with low prevalence of infection.
Individuals who test negative and continue to experience COVID-like symptoms of fever, cough and/or shortness of breath may still have SARS-CoV-2 infection and should seek follow up care from their healthcare provider. Individuals should provide all results obtained with this product to their healthcare provider for public health reporting. All healthcare providers will report all test results they receive from individuals who use the authorized product to relevant public health authorities in accordance with local, state, and federal requirements using appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LVID) Test Code Mapping for SARS-CoV-2 Tests provided by CDC.
The CareStart™ COVID-19 Antigen Home Test is authorized for non-prescription self-use and/or, as applicable for an adult lay user testing another person aged 2 years or older. The CareStart™ COVID-19 Antigen Home Test is only for use under the Food and Drug Administration’s Emergency Use Authorization.
The CareStart™ COVID-19 Antigen Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigens from SARS-CoV-2.
- Fast and easy to self-test at anywhere
- Easy to interpret the results using mobile application
- Qualitatively detect the SARS-CoV-2 nucleocapsid protein
- Use for nasal swab specimen
- Fast results only in 10 minutes
- Identify individual’s current infection status to COVID-19
For use under Emergency Use Authorization Only
Compatible OS System for Mobile Application:
- iOS 13 or newer for Apple iPhone
- Android 10 or newer for Android Phone
Disclaimer:
- As of November 22, 2021, CareStart™ COVID-19 Antigen Home Test is authorized to use with individual anterior nasal swab specimens from individuals age 14 years and older (self-collected), or 2 years and older (collected with adult assistance) for non-prescription home use by FDA under the EUA (EUA210314/S002)
- On June 7, 2022, FDA authorized the new labeling for CareStart™ COVID-19 Antigen Home Test (Quick Reference Instructions (QRI) and Fact Sheet for Individuals, Instructions for Use (IFU) for Healthcare Providers (HCP), Fact Sheet for HCP, and kit package); however, CareStart™ COVID-19 Antigen Home Test will continue to be distributed with the labeling previously authorized on the FDA’s enforcement discretion in efforts to increase the testing accessibility for COVID-19. Since the differences between the CareStart™ COVID-19 Antigen Home Test kit previously authorized (EUA210314/S002) and currently authorized (EUA210314/S007) are limited to the authorized labeling, you may use the CareStart™ COVID-19 Antigen Home Test kit with the intended use as indicated.
- Test Devices
- Extraction Vials and Caps
- Nasal Swab
- User Instructions with Fact Sheet for Individuals